good news on the Fuel Cell front
Platinum-nanoparticle-based catalyst could lead to low-cost, stable fuel cells
August 4, 2010 by Editor
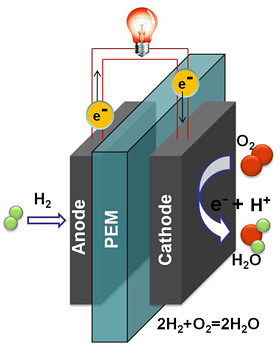
Fuel cells work by electrochemically decomposing fuel instead of burning it, converting energy directly into electricity.
Researchers at Cornell University's Energy Materials Center have discovered a catalyst — platinum nanoparticles — that could make fuel cells more stable, longer lasting, and more resistant to carbon monoxide poisoning.
Hydrogen fuel cells offer an appealing alternative to gasoline-burning cars: they have the potential to power vehicles for long distances using hydrogen as fuel, mitigate carbon dioxide production and emit only water vapor. But they also require very pure hydrogen to work. That means that conventional fuel must be stripped of its carbon monoxide (CO) — a process that is too expensive to make fuel cells commercially viable.
To create a catalyst that can tolerate more CO, the researchers deposited platinum nanoparticles on a support material of titanium oxide (with added tungsten to increase its electrical conductivity).
Tests show that the new material works with fuel that contains as much as 2 percent CO, losing only 5 percent efficiency compared with a 30 percent drop in efficiency for conventional platinum catalysts. The material is also more stable and less expensive than pure platinum.
The research was supported by the U.S. Department of Energy and by the Energy Materials Center at Cornell, an Energy Frontier Research Center funded by the Department of Energy.
More info: Cornell University news


0 Comments:
Post a Comment
<< Home